ANDA Approval: What It Means for Generic Drugs and Your Pocketbook
When you pick up a generic pill at the pharmacy, chances are it got through something called ANDA approval, a process the U.S. Food and Drug Administration uses to approve generic versions of brand-name drugs. Also known as the Abbreviated New Drug Application, this system lets companies prove their generic version works just like the original—without repeating every single clinical trial. It’s not just paperwork. ANDA approval is what keeps prices low, gives you choices, and ensures that the $4 version of your blood pressure pill does the same job as the $150 brand.
Behind every approved generic is a strict checklist: the active ingredient must match exactly, the strength and dosage form must be identical, and the drug must be absorbed into your body at the same rate and amount. The FDA doesn’t just trust the company’s word—they test samples, inspect manufacturing sites, and check for consistency batch after batch. This is why a generic from one company isn’t just "similar" to another—it’s legally and scientifically equivalent. That’s also why you can swap between different generic brands without worry. The same rules apply whether it’s a simple antibiotic or a complex heart medication. And if you’re wondering why some generics cost more than others? That’s not about quality—it’s about competition, supply chains, and how many makers are in the game.
ANDA approval doesn’t just affect your wallet. It connects directly to how safe your meds are. When a generic gets approved, the FDA also reviews its inactive ingredients—the fillers, dyes, and binders. That’s why some people react to one generic but not another: it’s not the active drug, it’s the extras. This is why posts on active vs inactive drug ingredients matter so much. It’s also why drug safety alerts, official warnings from the FDA and ISMP about potential risks in medications often include generic versions. And when you hear about insurance formulary tiers, how health plans group drugs by cost and coverage, you’re seeing ANDA approval in action—lower-tier generics are there because they’ve passed this bar.
What you’ll find in the posts below isn’t just theory. It’s real-world impact. From how single-source vs multi-source drugs, whether a drug has only one maker or many competing generics affect your out-of-pocket costs, to how medication safety updates, changes in drug guidelines from trusted health organizations can shift which generics are recommended, every article ties back to this one system: ANDA approval. It’s the quiet engine behind your prescriptions. And once you understand it, you’ll know exactly why your doctor says "it’s the same thing"—and when you should ask for more.
Bioequivalence Studies: What the FDA Requires Generic Drug Manufacturers to Prove
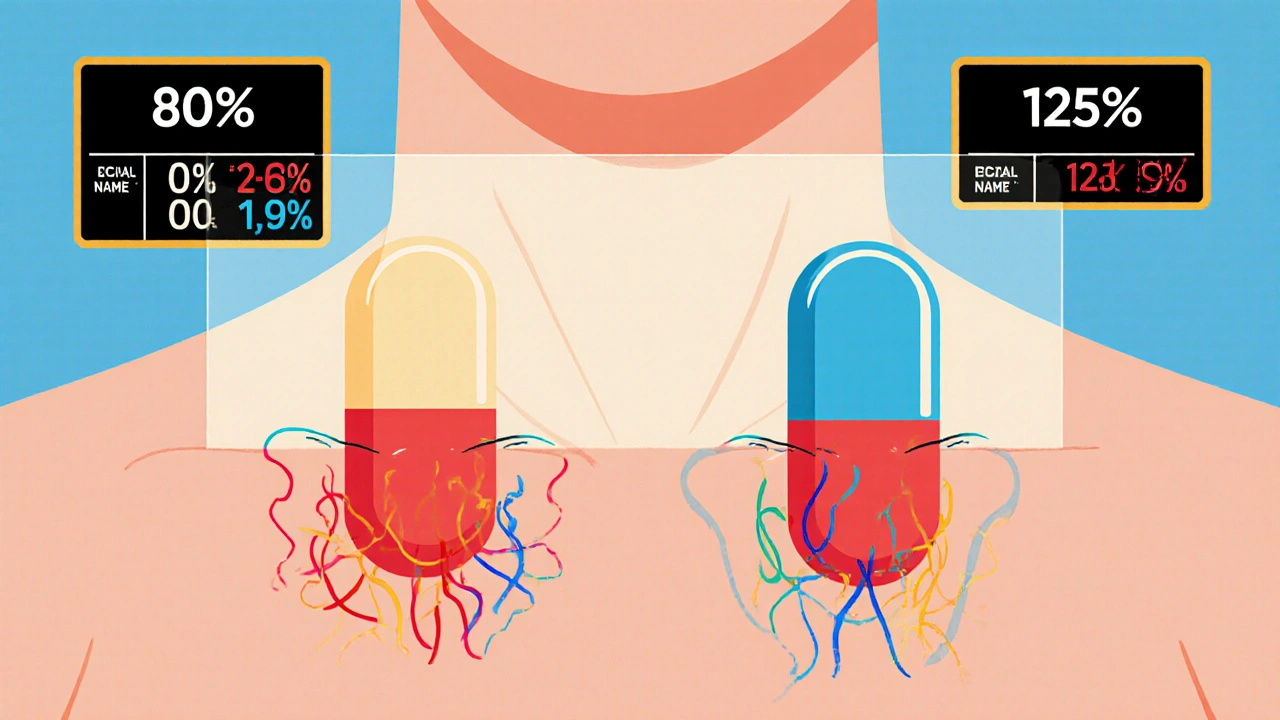
The FDA requires generic drug manufacturers to prove bioequivalence through rigorous studies showing their product absorbs at the same rate and extent as the brand-name drug. Learn the 80/125 rule, biowaivers, NTID exceptions, and why this matters for patient safety.
- November 27 2025
- Tony Newman
- 12 Comments
