Bioequivalence Criteria: What Makes Generic Drugs Truly Equal
When you pick up a generic pill, you’re trusting that it will do the same job as the brand-name version. That trust isn’t guesswork—it’s built on bioequivalence criteria, a set of strict scientific standards that prove a generic drug releases the same amount of active ingredient into your bloodstream at the same rate as the original. Also known as pharmaceutical equivalence, these criteria are the backbone of every generic drug approval worldwide. Without them, there’d be no way to know if a cheaper pill actually works the same way. It’s not just about cost—it’s about safety, consistency, and your health.
For a generic to pass bioequivalence testing, it must match the brand-name drug in two key ways: how much of the drug gets into your blood (bioavailability, the proportion of the drug that enters circulation and becomes active in the body), and how fast it gets there. Regulators like the FDA and EMA don’t just accept claims—they require real human studies. Volunteers take both versions under controlled conditions, and blood samples are taken over hours to map out the absorption curve. If the generic’s curve falls within 80% to 125% of the brand’s, it’s considered bioequivalent. That’s not a broad range—it’s a tight window designed to ensure no meaningful difference in how your body responds.
But bioequivalence isn’t just about the active ingredient. It also considers how the drug is delivered—tablet, capsule, or liquid—and whether excipients (inactive ingredients) interfere with absorption. That’s why a generic version of a drug made for slow release can’t just be a regular tablet chopped in half. The pharmaceutical equivalence, the match in dosage form, strength, route of administration, and inactive ingredients matters just as much. Even small changes in fillers or coatings can alter how quickly the drug dissolves, and that’s enough to throw off bioequivalence.
These rules aren’t theoretical. They’ve prevented real harm. In the 1980s, a generic version of an epilepsy drug failed to meet bioequivalence standards, leading to breakthrough seizures in patients. Since then, regulators have tightened testing—especially for drugs with narrow therapeutic windows, like warfarin, lithium, or cyclosporine. Even if two generics look identical on the shelf, they might not be interchangeable unless each has been individually proven bioequivalent to the brand. That’s why your pharmacist can’t always swap generics without checking the specific product code.
What you’re seeing in this collection of articles isn’t just random drug info—it’s the practical fallout of bioequivalence rules. From how insurance tiers decide which generics to cover, to why some people report different side effects with generics, to how single-source drugs stay expensive while multi-source ones drop in price—everything ties back to whether the drug in your hand meets the same bioequivalence standard as the one your doctor prescribed. You’ll find posts that explain how to tell if a reaction is from the drug itself or from an inactive ingredient, how to track safety updates on generics, and why some people need to stick with one brand even when generics are available. This isn’t about chemistry alone. It’s about real people, real outcomes, and the quiet science that keeps millions of prescriptions safe and effective every day.
Bioequivalence Studies: What the FDA Requires Generic Drug Manufacturers to Prove
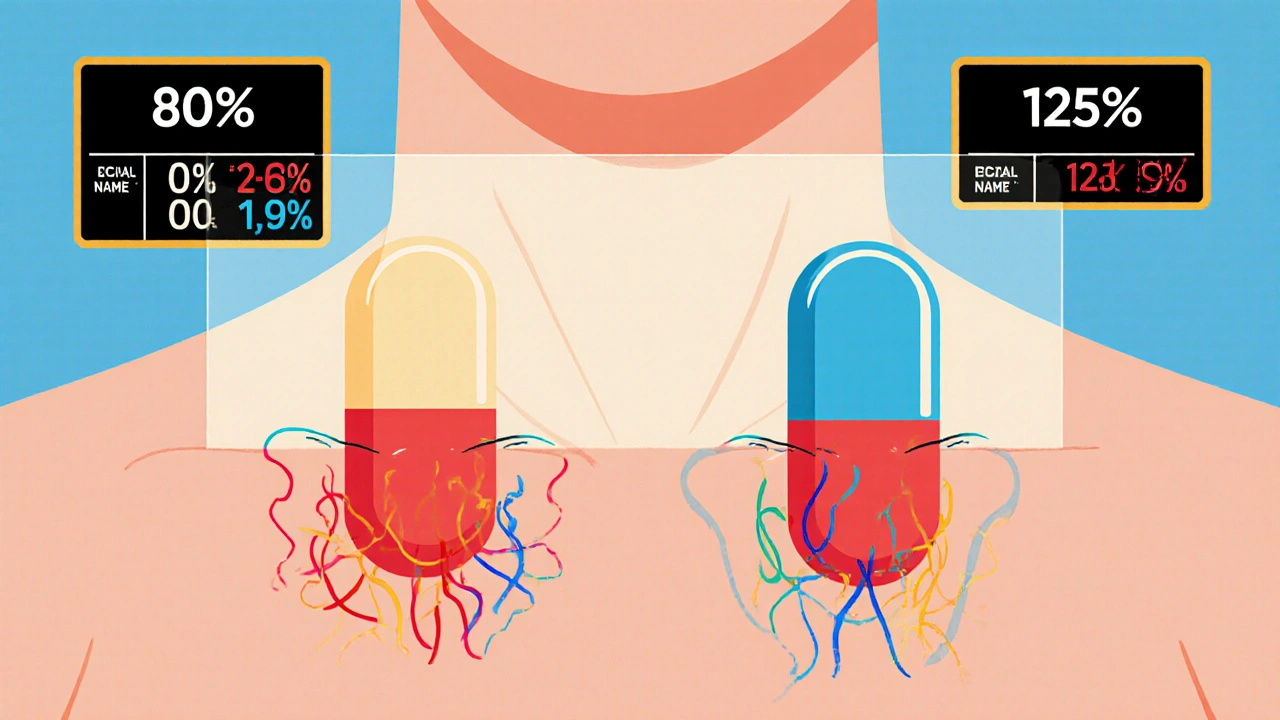
The FDA requires generic drug manufacturers to prove bioequivalence through rigorous studies showing their product absorbs at the same rate and extent as the brand-name drug. Learn the 80/125 rule, biowaivers, NTID exceptions, and why this matters for patient safety.
- November 27 2025
- Tony Newman
- 12 Comments
