Bioequivalence Studies: What They Are and Why They Matter for Generic Drugs
When you pick up a generic pill, you expect it to do the same job as the brand-name version—and bioequivalence studies, controlled tests that prove two drug formulations release the same amount of active ingredient at the same rate in the body. These studies are the reason you can save money without sacrificing effectiveness. Without them, generic drugs would just be copies in name only. The FDA and other global regulators require these tests before a generic version can be sold. It’s not about price—it’s about proof.
Generic drugs, medications with the same active ingredient, strength, and dosage form as brand-name versions aren’t cheaper because they’re weaker. They’re cheaper because their makers didn’t pay for the original research. But to be approved, they must match the original in how quickly and completely the body absorbs the drug. That’s where drug absorption, the process by which a medication enters the bloodstream after being taken becomes critical. A generic drug might look identical, but if it’s absorbed too slowly or too fast, it won’t work the same way. Bioequivalence studies measure blood levels over time to make sure the curve matches the brand-name drug within strict limits—usually within 80% to 125% of the original.
These studies aren’t done on patients with the disease. They’re usually done on healthy volunteers under controlled conditions. The same dose is given, and blood samples are taken over hours to track how the drug moves through the body. This isn’t guesswork—it’s science. And it’s why millions of people safely switch to generics every day. You might not realize it, but if you’ve ever filled a prescription for metformin, lisinopril, or atorvastatin as a generic, you’re benefiting from these tests.
Not all generics are created equal, though. Some brands make multiple versions, and sometimes one version passes bioequivalence while another doesn’t. That’s why your pharmacist might switch your generic without telling you—and why you should pay attention if your symptoms change after a switch. Pharmaceutical equivalence, when two drugs contain identical amounts of the same active ingredient in the same dosage form is just the starting point. The real test is whether your body responds the same way.
These studies also explain why some generics work better for you than others. If you’ve ever felt a difference after switching brands, it’s likely due to inactive ingredients—fillers, coatings, or dyes—that affect how the drug breaks down. Bioequivalence studies focus on the active ingredient, but your body doesn’t care about labels—it cares about results. That’s why some people stick to one generic brand even when others are cheaper.
What you’ll find in the posts below are real-world stories and facts about how drugs behave in your body, what happens behind the scenes when a generic hits the shelf, and how to spot when a switch might be risky. You’ll learn about the hidden factors that affect how your meds work, why some drugs can’t be easily copied, and how to talk to your doctor if something feels off. This isn’t theory. It’s what happens when science meets your medicine cabinet.
Bioequivalence Studies: What the FDA Requires Generic Drug Manufacturers to Prove
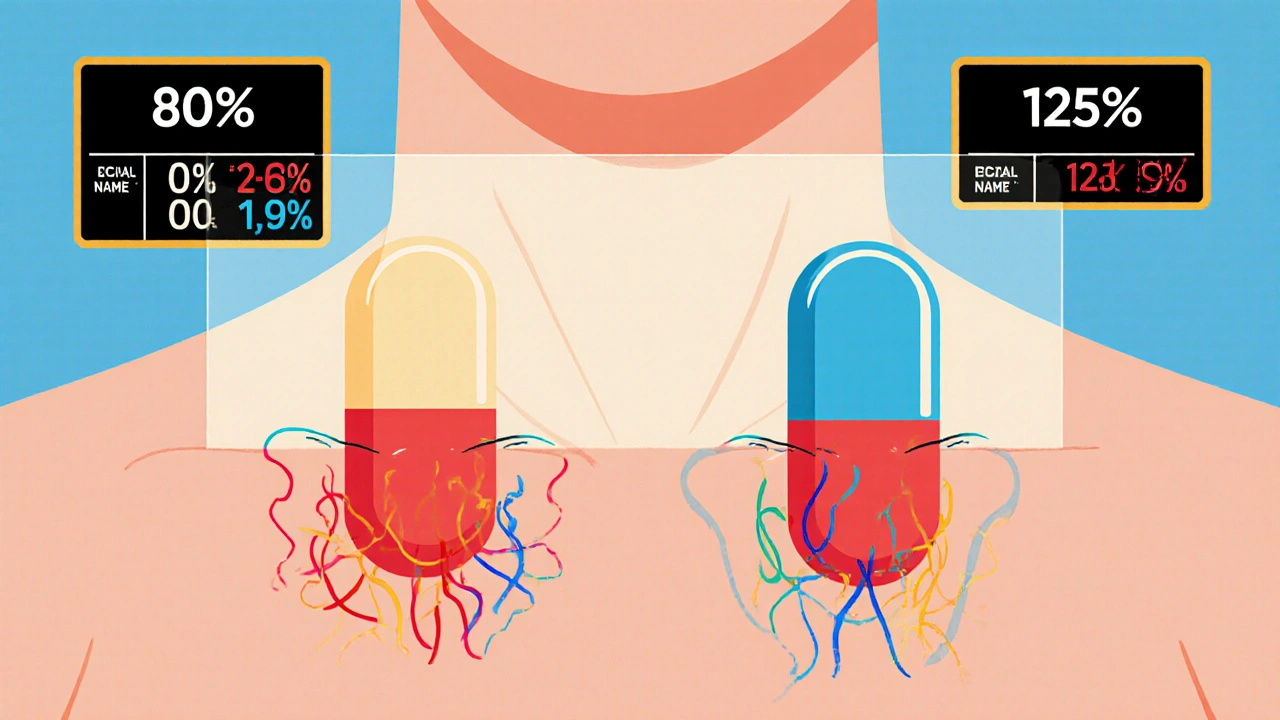
The FDA requires generic drug manufacturers to prove bioequivalence through rigorous studies showing their product absorbs at the same rate and extent as the brand-name drug. Learn the 80/125 rule, biowaivers, NTID exceptions, and why this matters for patient safety.
- November 27 2025
- Tony Newman
- 12 Comments
