TNF Inhibitor TB Risk Comparison Tool
TNF Inhibitor Risk Comparison
Risk levels based on clinical studies showing TB reactivation rates
Why Risk Levels Differ
These drugs work differently on the immune system:
- Adalimumab & Infliximab: Monoclonal antibodies that block both free and cell-bound TNF-alpha. This disrupts the granuloma walls that contain TB, increasing reactivation risk by 3-4 times compared to etanercept.
- Etanercept: Binds only to free TNF-alpha in the blood, leaving cell-bound TNF-alpha intact. This preserves granuloma structure and maintains TB containment, resulting in lower TB risk.
Key Insight: TNF-alpha is critical for maintaining immune walls around TB bacteria. Drugs that disrupt cell-bound TNF-alpha (like adalimumab) pose higher TB reactivation risk than those that only target free TNF-alpha (like etanercept).
Critical Screening & Monitoring
When you start a TNF inhibitor like adalimumab or infliximab for rheumatoid arthritis or Crohn’s disease, you’re getting powerful relief from inflammation. But behind that relief is a hidden risk: tuberculosis. Not the kind you catch from a cough in a crowded bus - the kind that’s been sleeping in your lungs for years, quietly waiting for your immune system to weaken. That’s what TNF inhibitors can trigger. And it’s not rare. In some clinics, one in every 70 patients on these drugs ends up with active TB, even if they were told they were clear before starting.
Why TNF Inhibitors Wake Up TB
Your body keeps tuberculosis under control using tiny structures called granulomas. These are like immune system prisons - walls of cells that trap the TB bacteria and stop them from spreading. TNF-alpha is the glue that holds those walls together. When you block TNF-alpha with drugs like adalimumab or infliximab, those walls start to crumble. The bacteria wake up, multiply, and escape into your bloodstream. Not all TNF inhibitors are the same. Etanercept works differently. It’s a soluble receptor that soaks up excess TNF-alpha in the blood, but it doesn’t stick to the TNF on the surface of immune cells. That means it leaves the granuloma walls mostly intact. That’s why patients on etanercept have less than a quarter of the TB risk compared to those on antibody-based drugs like adalimumab and infliximab. Studies show the risk is 3 to 4 times higher with those two.Screening Isn’t Optional - It’s Non-Negotiable
Before you get your first TNF inhibitor shot or infusion, you need two things: a TB test and a clear history. The two standard tests are the tuberculin skin test (TST) and the interferon-gamma release assay (IGRA). Neither is perfect. TST can give false positives if you’ve had the BCG vaccine - common in many countries. IGRA is more specific but costs more and isn’t available everywhere. In high-TB-burden countries like India, the Philippines, or parts of Africa, guidelines say: treat for latent TB even if the test is negative. Why? Because the tests miss up to 20% of infections. In the U.S., where TB is rare, the risk is lower, but not zero. A study of over 7,000 patients on TNF inhibitors found two cases of active TB - even though 80% had been screened and treated. The bottom line: if you’re from a country where TB is common, or you’ve lived in one, been in close contact with someone who had TB, or worked in a hospital or prison - you need treatment for latent TB before starting your biologic. The standard is 9 months of isoniazid. But many patients drop out because of liver side effects. Newer regimens like 4 months of rifampin and isoniazid are now approved and improve adherence by nearly 30%.What Happens If You Skip Screening?
Clinics that skip screening don’t just take a risk - they gamble with lives. A review of 1,200 patients across five rheumatology centers found that 18% of those who developed TB had negative screening results before starting treatment. That’s not a failure of the test - it’s a failure of assumption. Some people get infected right before starting the drug. Others have false negatives because their immune system is already weakened by the disease they’re treating. One case from a Melbourne clinic involved a 54-year-old woman from Vietnam. Her TST was negative. She started adalimumab for psoriatic arthritis. Three months later, she was in the ICU with TB meningitis. Her CT scan showed dozens of tiny nodules in her brain. She survived, but barely. Her doctors later realized she’d had a recent exposure to TB at a family gathering - something she didn’t think was important to mention.
Monitoring After Starting Treatment
Screening isn’t the end. You need ongoing checks. For the first year on a TNF inhibitor, you should be asked every three months: Have you had fever? Night sweats? Unexplained weight loss? A cough that won’t go away? These aren’t just symptoms - they’re red flags. Most TB cases in TNF inhibitor users aren’t in the lungs. In fact, 78% are extrapulmonary - meaning they show up in the spine, brain, kidneys, or lymph nodes. That makes diagnosis harder. A simple chest X-ray won’t catch TB in your spine. You might need an MRI, a biopsy, or a spinal tap. Delayed diagnosis means worse outcomes. There’s also a rare but dangerous twist: TB-IRIS. That’s when your immune system, after being suppressed by the TNF inhibitor, suddenly wakes up - and overreacts to the TB bacteria you’re now treating. It causes fever, swelling, and sometimes organ damage. It usually hits 2 to 4 months after you start TB treatment. Doctors need to recognize it fast - and often use steroids to calm the immune system down.The Real-World Gap
In wealthy countries like Australia, the U.S., or Germany, most clinics follow guidelines. But globally, that’s not the case. A WHO survey in 2023 found that 80% of rheumatology clinics in low-income countries don’t have access to IGRA tests. Many rely on TST alone - which is unreliable if you’ve had BCG. In these places, the risk of TB reactivation is much higher, and death rates are 23% greater than for regular TB cases. Even in places with good access, human error happens. A 2022 survey of U.S. rheumatologists found that 27% of patients had their treatment delayed because their TB treatment wasn’t properly documented. One patient waited six months for her adalimumab because her old TB treatment records were lost. Another was started on the drug too soon after finishing isoniazid - just 2 weeks later. She developed TB within 8 weeks.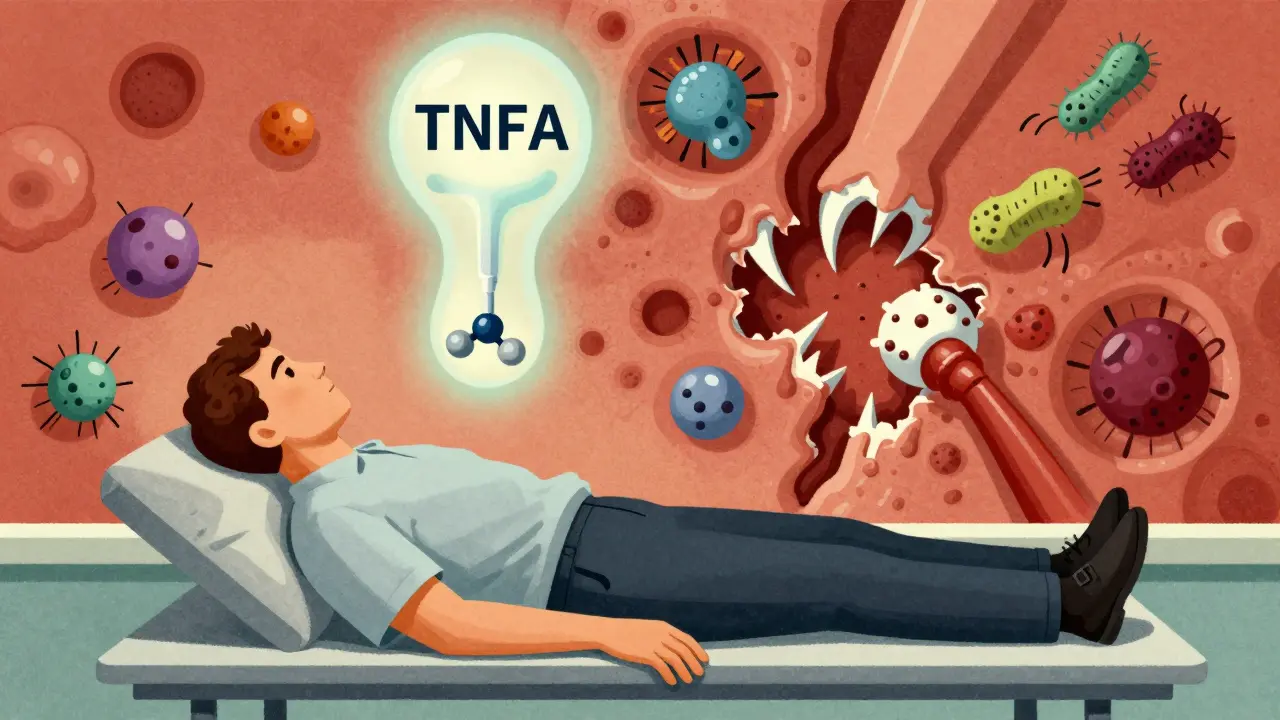
What’s Next? Safer Drugs on the Horizon
Researchers are working on next-generation TNF inhibitors that don’t break down granulomas. Early animal studies show promise. A new drug targeting CD271 - a protein involved in TNF signaling - reduced TB reactivation by 80% compared to traditional drugs. It’s still in Phase II trials, but if it works in humans, it could change everything. In the meantime, we’re stuck with what we have. The safest approach is simple: screen everyone. Treat those with latent TB. Monitor closely. Don’t assume negative tests mean zero risk. And never start a TNF inhibitor without knowing the patient’s TB history - even if they’re from Australia.What You Should Do Right Now
If you’re considering a TNF inhibitor:- Ask for both TST and IGRA - especially if you’ve lived abroad or had close contact with someone with TB.
- Confirm you’ve had at least 1 month of LTBI treatment before starting your biologic.
- Know your drug: etanercept has lower TB risk than adalimumab or infliximab.
- Report any fever, night sweats, or weight loss immediately - even if it’s months after starting.
- Keep a copy of your TB treatment records. Hospitals lose them. You shouldn’t.
- Don’t skip your quarterly check-ins. Those questions about cough and sweat aren’t just busywork.
- If you travel to a high-TB country, tell your doctor. You may need extra monitoring.
- If you’re pregnant or planning to be, discuss TB risk - it can affect your baby.
FAQ
Can I get TB even if my screening test was negative?
Yes. Up to 20% of people with latent TB test negative on TST or IGRA. This can happen if your immune system is weak from your autoimmune disease, if you were recently exposed, or if the test was done too soon after exposure. That’s why doctors still recommend treating latent TB before starting TNF inhibitors - even if tests are negative - especially if you’re from a high-risk country.
Why is adalimumab riskier than etanercept for TB?
Adalimumab and infliximab are monoclonal antibodies that bind to both free and cell-bound TNF-alpha. This disrupts the granulomas that keep TB dormant. Etanercept only binds to free TNF-alpha, leaving the cell-bound version intact - which is critical for maintaining those immune walls. That’s why the risk of TB reactivation is 3 to 4 times higher with adalimumab and infliximab.
How long after starting TNF inhibitors does TB usually appear?
Most cases occur within the first 3 to 6 months after starting the drug, especially with infliximab. But TB can develop anytime - even after years of treatment. That’s why ongoing monitoring is required for as long as you’re on the medication.
Is it safe to stop TNF inhibitors if I develop TB?
Yes - and you must. If you develop active TB, your TNF inhibitor must be stopped immediately. You’ll need at least 2 weeks of anti-TB treatment before restarting any biologic. Restarting too soon can cause TB to come back worse. Your doctor will monitor you closely before deciding if it’s safe to go back on the drug.
Can I get a TB vaccine (BCG) while on a TNF inhibitor?
No. The BCG vaccine is a live, weakened form of TB bacteria. If your immune system is suppressed by a TNF inhibitor, you could develop a serious infection from the vaccine itself. Always get BCG before starting any biologic - not after. If you’re unsure if you’ve had it, don’t assume. Your doctor can check your records or do a test.

Phil Hillson
January 18, 2026 AT 15:31So let me get this straight we’re giving people drugs that can wake up a sleeping killer just to stop their joints from aching
And we act surprised when someone ends up in the ICU with TB meningitis
That’s not medicine that’s Russian roulette with a prescription
I’ve seen clinics skip screening because it’s ‘too much paperwork’
And now people are dying because someone didn’t want to check a box
Why are we even still using these drugs if the risk is this high
Jacob Hill
January 19, 2026 AT 18:47Actually, I think this is a really well-researched piece-and I say that as someone who’s been on adalimumab for five years. The granuloma explanation was spot-on. I had my IGRA and TST done, got the 9-month isoniazid, and waited the full month before starting. My rheumatologist even called my old clinic to confirm my records. It’s annoying, yes-but it’s not optional. If you’re going to take a biologic, you owe it to yourself to do the due diligence. Also, etanercept is a legit safer option if your insurance covers it. I switched after my first TB scare (negative test, but I’m from Texas near the border-still got treated just in case).
Astha Jain
January 20, 2026 AT 09:11lol so u know in india we dont even have igra tests in most places... tst is all we got and bcg is given to evryone at birth so its useless
my doc just said 'u r from india so u get isoniazid no matter what' and i was like ok
but then i read this and realized i was lucky
my cousin's uncle got tb after adalimumab and died in 3 weeks
no one even asked about his travel history
we just assumed he 'got sick'
so yeah... this is real
and its not being talked about enough
Malikah Rajap
January 22, 2026 AT 07:31It’s so wild to me how we treat autoimmune diseases like they’re just a glitch in the system that needs fixing, but we forget that the immune system isn’t just a machine-it’s a living, breathing network of intelligence...
And when we mess with TNF-alpha, we’re not just blocking inflammation-we’re disrupting ancient, evolved defenses that kept our ancestors alive for millennia.
TB isn’t some relic-it’s a silent partner in our evolutionary story. And now we’re waking it up like we’re playing with fire in a library.
Maybe the real question isn’t how to screen better... but whether we should be suppressing the immune system at all.
Just saying.
Also, I’ve had night sweats since I started my drug-should I be worried? I’ve been meaning to ask my doctor but... I don’t want to sound paranoid.
sujit paul
January 23, 2026 AT 08:05It is a matter of profound concern that the medical establishment, despite possessing unequivocal evidence regarding the reactivation risk of TNF inhibitors, continues to rely on fallible diagnostic modalities such as the TST and IGRA, which are demonstrably inadequate in populations with prior BCG vaccination or latent exposure.
Furthermore, the commercial interests of pharmaceutical entities must be scrutinized, as the promotion of high-risk biologics like adalimumab over safer alternatives such as etanercept appears to be driven by profit margins rather than patient safety.
It is imperative that global health policy be restructured to mandate universal pre-treatment prophylaxis in all individuals with any history of residence in endemic regions-regardless of test results.
Failure to do so constitutes a breach of the Hippocratic Oath.
Lydia H.
January 24, 2026 AT 17:03My mom’s on etanercept and she’s from the Philippines. She had a negative TST but got the 4-month rifampin regimen anyway. She said the side effects were way easier than isoniazid-no liver issues, just a weird orange pee (which she joked was her new fashion statement).
And honestly? The quarterly check-ins? They’re not just busywork. Last year she mentioned a cough that wouldn’t go away and they found a tiny lymph node-turned out to be nothing. But if she hadn’t said anything? Who knows.
It’s scary, but if you stay awake and ask questions, you’re already ahead of most people.
Josh Kenna
January 26, 2026 AT 01:59They’re all just trying to sell you drugs. The real reason they push adalimumab over etanercept? It’s more expensive. Insurance pays more. Doctors get kickbacks. I’m not saying that’s true everywhere-but I’ve seen it. My cousin’s rheumatologist literally said, ‘We’re gonna start you on Humira-it’s the gold standard.’ When I asked why not etanercept, he got defensive. And now she’s on TB meds. Thanks, gold standard.
And don’t even get me started on how hospitals lose records. I had to beg for my own TB treatment docs for six months. One nurse said, ‘We don’t keep those for patients who aren’t in our system anymore.’
That’s not a system. That’s a dumpster fire.
Erwin Kodiat
January 26, 2026 AT 11:29Just wanted to say-this post saved my life. I’m from Nigeria, moved to the US 5 years ago. Got diagnosed with psoriatic arthritis. My doctor wanted to start me on infliximab. I asked about TB. He said, ‘You’re fine, you’ve been here five years.’ I pushed. Got an IGRA. Positive. Had latent TB. Treated it. Now I’m on etanercept. I’m alive. And I’m not letting anyone tell me I’m overreacting.
For anyone reading this from a high-risk country: don’t let anyone gaslight you. You’re not paranoid. You’re prepared.
And if you’re a doctor reading this? Please stop assuming. Ask. Listen. Save lives.